Absolute vs Gauge Pressure
Explain the differences between absolute, atmospheric, and gauge pressure. What about vacuum gauge pressure?
Expand Hint
Pressure can be described as the force applied to an area:
$$$P=\frac{F}{A}$$$
Hint 2
Atmospheric pressure (barometric pressure) is the pressure within Earth’s atmosphere, which is defined as 101 kPa (or 14.7 psi).
Pressure can be described as the force applied to an area:
$$$P=\frac{F}{A}$$$
Atmospheric pressure (barometric pressure) is the pressure within Earth’s atmosphere, which is defined as 101 kPa (or 14.7 psi). This standard atmosphere pressure unit (symbol: atm) is roughly equivalent to the average sea level atmospheric pressure on Earth, so the Earth’s atmospheric pressure at sea level is ~1 atm. The pressure relative to atmospheric pressure is called Gauge pressure. Gauge pressure is positive for pressures above atmospheric pressure, and negative for pressures below it. Negative gauge pressure is called vacuum gauge pressure. Absolute pressure is measured relative to the absolute zero pressure point, and is the sum of gauge pressure and atmospheric pressure:
$$$P_{abs}=P_{atm}+P_{gauge}$$$
$$$P_{abs}=P_{atm}-P_{vacuum\:gauge}$$$
For example, if a car’s tire gauge reads 30 psi, then the absolute pressure is 30 psi + 14.7 psi = 44.7 psi.
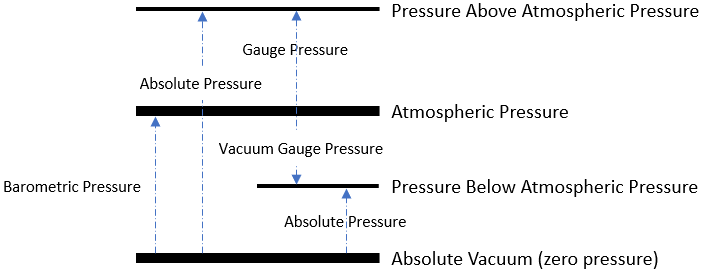
As an overview:
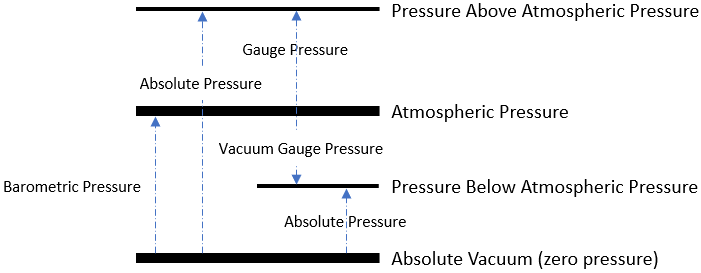
The simplest distinction between the gauge and absolute is that absolute pressure uses absolute zero as its standard baseline, while gauge pressure uses atmospheric pressure as its zero point.
Atmospheric pressure is the pressure within Earth’s atmosphere.
Time Analysis
See how quickly you looked at the hint, solution, and answer. This is important for making sure you will finish the FE Exam in time.- Hint: Not clicked
- Solution: Not clicked
- Answer: Not clicked
Similar Problems from FE Sub Section: The Pressure Field in a Static Liquid
014. Swimming Pool Pressure
297. Pressurized Pump Power
311. Water Tower
352. Pressure Difference
578. Water Height
601. A Pump’s Pressure
Similar Problems from FE Section: Characteristics of a Static Liquid
014. Swimming Pool Pressure
033. Archimedes' Principle
297. Pressurized Pump Power
311. Water Tower
352. Pressure Difference
578. Water Height
601. A Pump’s Pressure