Phase Diagram
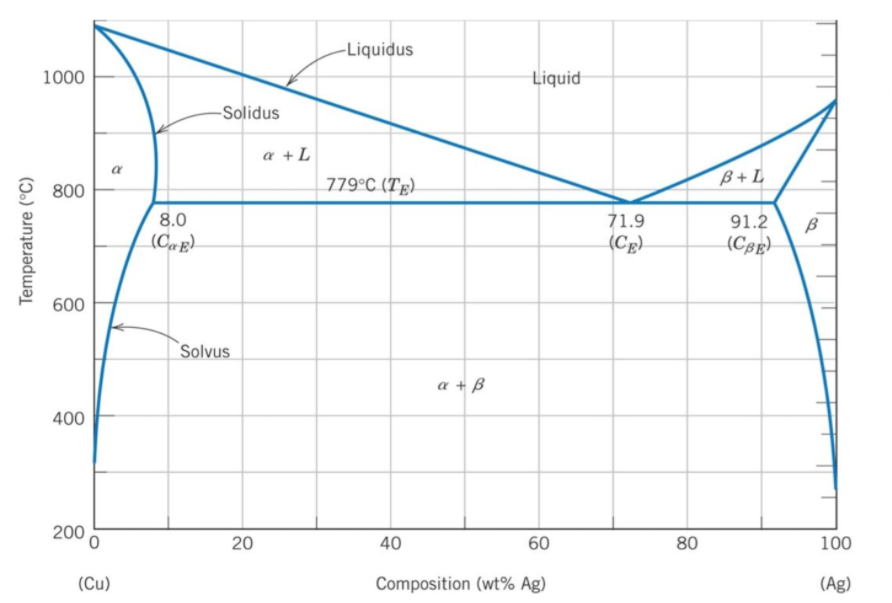
An alloy that is 80% silver by weight is fully melted and allowed to slowly cool. Using the binary phase diagram, what phase(s) are present at 800°C?
Expand Hint
Hint 2
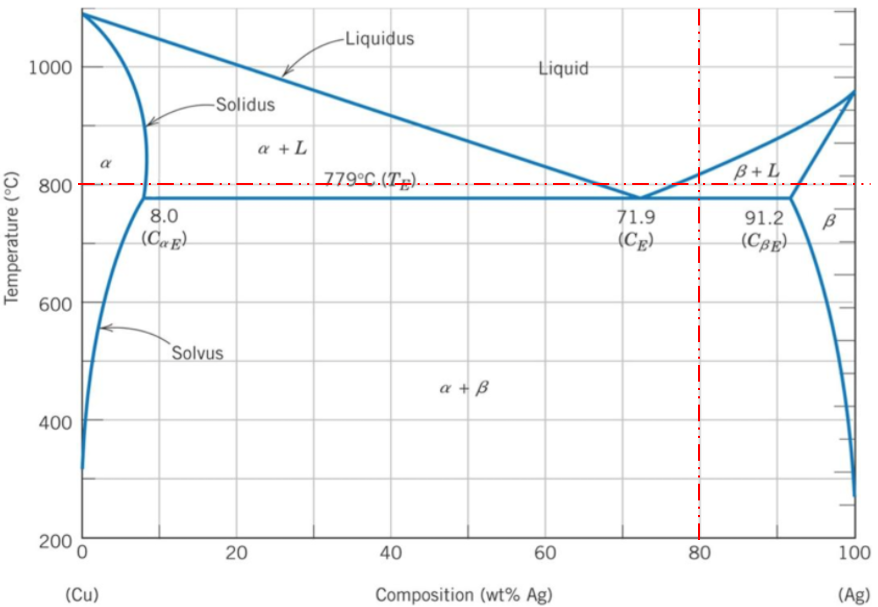
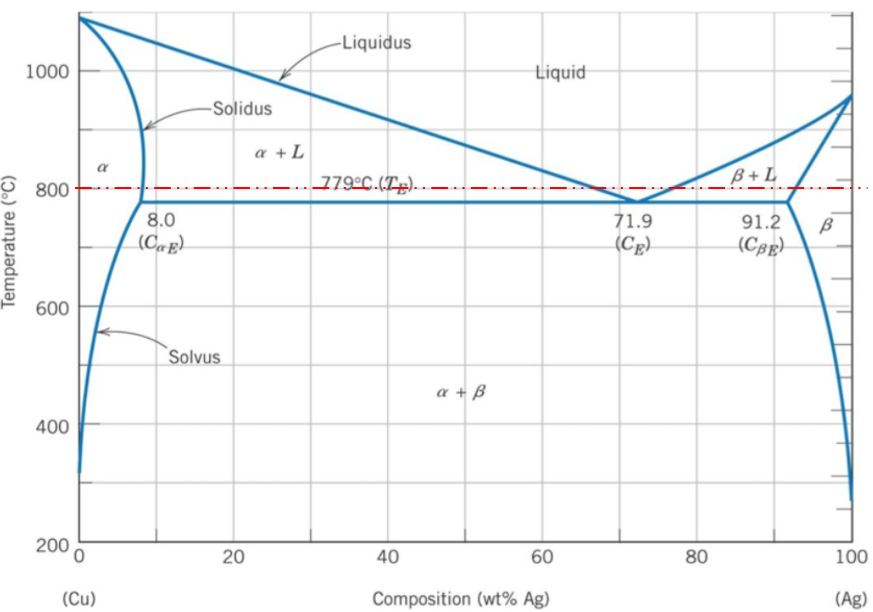
A binary phase diagram shows the phases (solid, liquid etc) formed by mixing two different elements over a range of temperatures. The phase diagram's x-axis represents the alloy's composition weight of silver. The y-axis represents the alloy's temperature. The 80% silver by weight intersects a 800°C temperature in the
$$\beta +L$$
zone, meaning
$$\beta +L$$
phases are present.
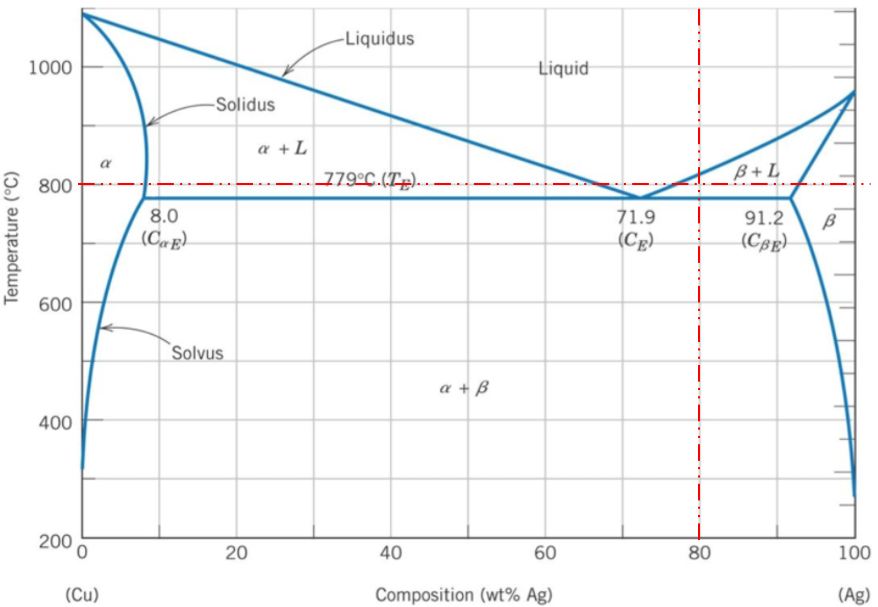
$$$\beta +L$$$
Time Analysis
See how quickly you looked at the hint, solution, and answer. This is important for making sure you will finish the FE Exam in time.- Hint: Not clicked
- Solution: Not clicked
- Answer: Not clicked
Similar Problems from FE Section: Binary Phase Diagrams
233. Eutectic Composition
372. Binary Phase Diagram